Explain the Major Differences Between Covalent and Ionic Bonding
7 rows Ionic and covalent bonds are fundamentally different in the way they are formed. 222aThe main differences between the various forms of primary bonding are.

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
A Briefly cite the main differences among the ionic covalent and metallic bonding.
. Covalent - A shared pair of electrons resulting in both atoms having full outer shells. Atoms that participate in an ionic bond have different electronegativity values from each other. Which bond ionic or covalent involves cations and anions.
Describe the differences between the three types of bonding. Formed by transfer of an electron from one atom to another. Covalent bond have low polarity while ionic bond has a high polarity.
The three different types of bonding are covalent ionic and metallic bonding. The main difference between the coordinate bond and an ionic bond is that an Ionic bond is formed when two oppositely charged ions are attracted in other words when there is an electrostatic attraction between two oppositely charged ions. Dinitrogen Hexafluoride Between two NONMETALS Sharing of Electrons Change endings of the last element to.
What is the name for N2F6. Formed by a sharing of electrons between two atoms. Therefore their bonding pattern can be deemed as the key difference between ionic and covalent compounds.
The main reason for these differences is the difference in their bonding pattern. Difference Between Ionic and Covalent Bonds When ionic bonds are formed electrons is donated by a metal and donated electrons is accepted by a non-metal. In a true covalent bond If the electron is shared equally between the atoms forming a covalent bond then.
Core Difference Between Covalent and Ionic Bonds. The difference between naming ionic covalent compounds Covalent Bonds Example. The compound has the low melting point.
Ionic is losing and gaining electrons Covalent is sharing the same electrons Ionic - One atom loses and electron the other gains one and two oppositely charged ions are produced which are attracted to each other. In general metallic elements tend to form ionic bonds and non-metallic elements tend to form covalent. In covalent bonds atoms are electrostatically attracted within the course of each other whereas in ionic bonds.
It involves the transfer of electrons from the metal to the non-metal producing a positively charged metal ion and negatively charged non-metal ion. Covalent bond occurs when atoms share their outer shell electrons with each other while ionic bond occurs when one atom donates an electron to another atom. Ionic bond involves complete loss or gain of pair of electrons between two atoms whereas in covalent bond only sharing of electrons takes place.
Explain the differences between ionic and covalent bonds by answering the following questions. The difference between ionic and covalent bond is that ionic bonds occur between atoms having very different electronegativities whereas covalent bonds occur between atoms with similar or very low electronegativity differences. Start studying Difference Between Ionic and Covalent Bonds.
Hydrogen bonding involves bonding of highly electronegative elements like fluorine oxygen and nitrogen with hydrogen. This leads to giant structures of metal atoms arranged in a regular pattern. The main difference between ionic bonds and covalent bonds is sharing of electron pairs and atoms.
9 rows Difference Between Ionic Covalent and Metallic bonds - The major difference between Ionic. The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. The ions then attract each other through electrostatic forces of attraction as they are oppositely charged.
How does the formation of cationanions in the bond. Covalent bonds involve sharing of electrons in the valence shell metallic bonds are the attraction between the delocalized electrons present in the lattice of the metals and ionic bonds are referred as the transferring and. Here participating atoms have a high electronegativity difference.
Learn vocabulary terms and more with flashcards games and other study tools. Here participating atoms have almost the same electronegativity. Covalent--there is electron shari.
Ionic bond has no definite shape while covalent bond has a definite shape. Ionic bonding occurs between metal and non-metal atoms. 7 rows An ionic bond essentially donates an electron to the other atom participating in the bond.
How do ionic bonds differ from covalent bonds when it comes to what happens to the electrons of the atoms involved in each type of bond. In a covalent bond the atoms are bound by the sharing of electrons. A compound has the high melting point.
Covalent bonding occurs when atomsmolecules share pairs of electronsMetallic bonding is bonding that occurs in metals. Ionic--there is electrostatic attraction between oppositely charged ions. If we talk about the differences between these two bonds we would have to go deep.
Electron pairs are shared between atoms. -In an ionic bond the atoms are bound together by the attraction between oppositely-charged ions-In a covalent bond the atoms are bound by shared electrons. Covalent bonds occur between two non-metals metallic bonds is between two metals while ionic is observed between non-metal and metal.
View the full answer.
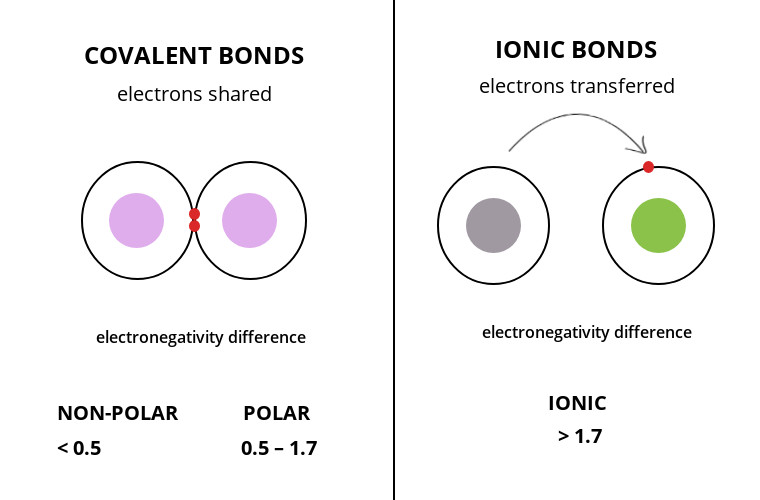
15 Major Difference Between Covalent And Ionic Bonds With Table Core Differences
Which Are Soluble In Water Covalent Compounds Or Ionic Compounds Quora
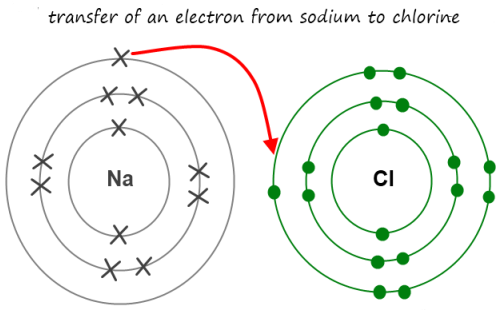
Covalent Vs Metallic Vs Ionic Bond General Differences And Examples Viva Differences
What S The Difference Between An Ionic Bond And A Covalent Bond Quora

Difference Between Ionic And Covalent Bonds Compare The Difference Between Similar Terms

College Biochemistry Major Ionic Bond Vs Covalent Bond Covalent Bonding Covalent Bonding Worksheet Ionic Bonding
Difference Between Covalent And Ionic Bonds

Ionic And Covalent Bonding Are Depicted In The Picture Ionic Bonds Is The Attraction Of A Cation To An An Ionic Bonding Teaching Chemistry Teaching Science
Difference Between Ionic Covalent And Metallic Bonds Definition Formation Properties
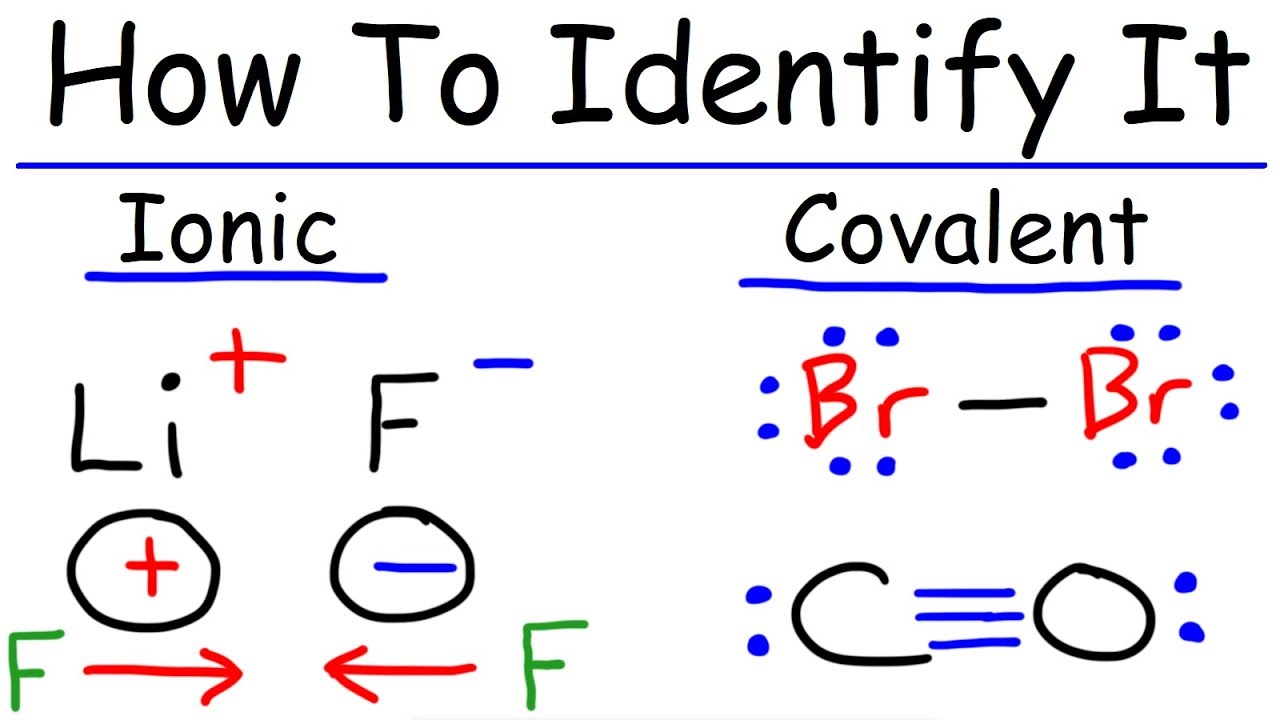
Major Differences Between Ionic And Covalent Compounds People Ignore Current School News

Difference Between Ionic And Covalent Bonds Compare The Difference Between Similar Terms

What S The Difference Between Ionic And Covalent Bonds
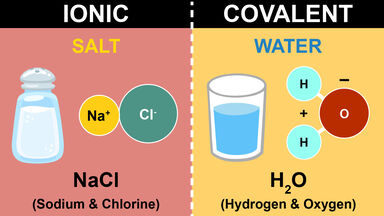
Main Differences Between Ionic And Covalent Bonds

Write 5 Points Of Difference Between Ionic And Covalent Compound
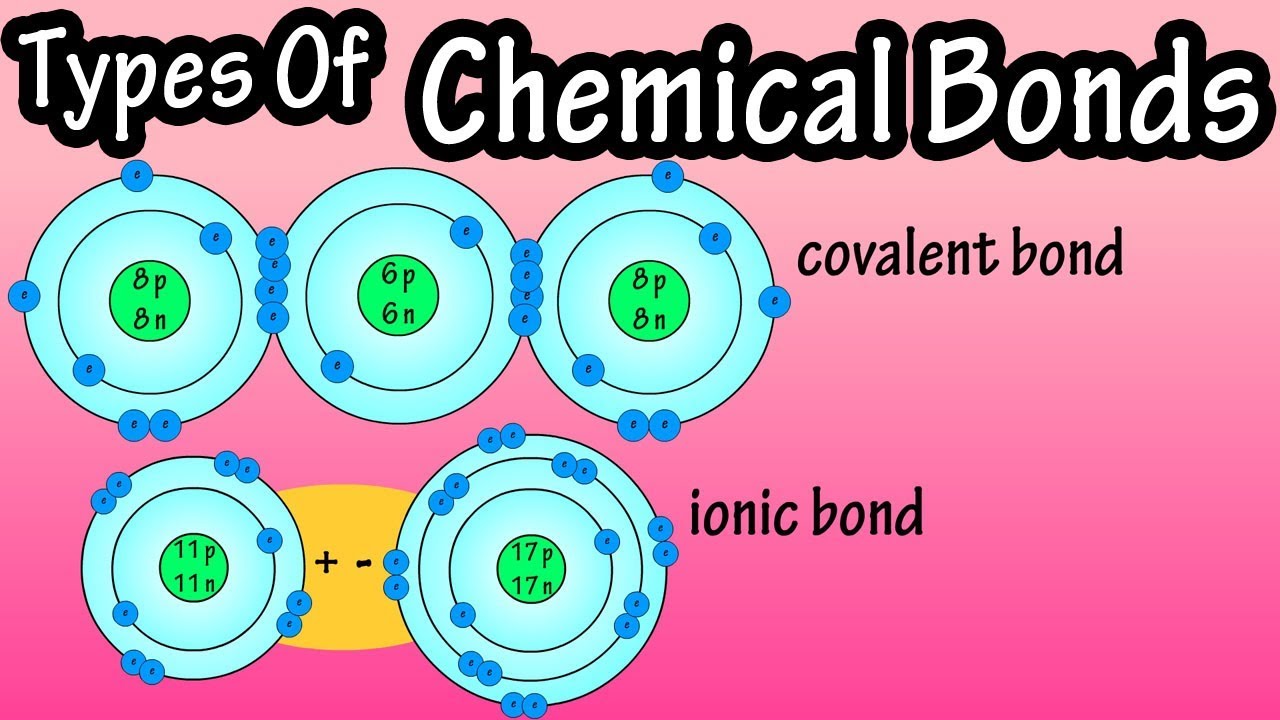
Types Of Chemical Bonds What Are Chemical Bonds Covalent Bonds And Ionic Bonds What Are Ions Youtube

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Ionic Covalent And Metallic Bonds Differences And Similarities

Comments
Post a Comment